Abstract
Introduction
The role of T cells in chemotherapy response and maintenance of remission in acute myeloid leukemia (AML) patients is not fully understood. In solid tumors and chronic infections, exhaustion is a multistep process ranging from less differentiated progenitor exhausted (Tpex) to intermediate and terminally exhausted T cells (Beltra et al. 2020). High frequencies of Tpex correlate with response to immune-checkpoint blockade in solid tumors (Miller et al. 2019). In AML, where the backbone of treatment is chemotherapy, the role of dysfunctional T-cell subsets has yet to be elucidated.
Methods
Serial bone marrow (BM) samples from 16 AML patients (10 complete responders (Res) and 6 non-responders (NonRes)) at diagnosis and at response assessment after induction chemotherapy and 12 healthy donors (HD) were analyzed by flow cytometry using a 13-color panel. Moreover, we performed single-cell RNA sequencing (scRNAseq) (10X Genomics) on BM samples from 2 HD and 5 AML patients (3 Res, 2 NonRes) at baseline and after chemotherapy. Subsequently, we used a scRNAseq-guided 26-color spectral flow cytometry panel and explored T-cell phenotypes on BM of 22 AML patients (12 Res and 10 NonRes). Custom-made R scripts were employed for high-dimensional flow cytometry and scRNAseq analysis.
Results
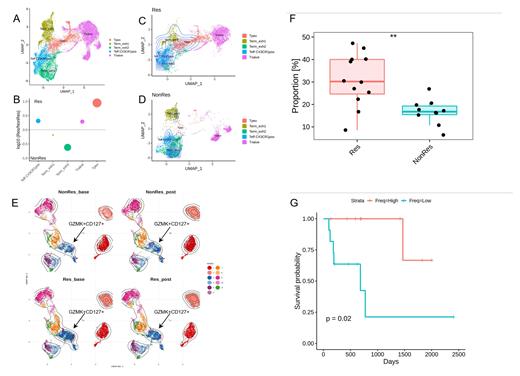
Initial flow-cytometry analysis showed a significant increase in BM PD1 +CD28 + CD8 + T cell subset (p<0.01) in Res vs NonRes at baseline and post-chemotherapy (data not shown). To further investigate these results, we performed 5' VDJ scRNAseq and used gene signatures mapped in two dimensions via UMAP to annotate the T-cell clusters as naive, Tpex, T effector CX3CR1 + (Teff CX3CR1pos), Terminally exhausted 1 (Term_exh1) and Terminally exhausted 2 (Term_exh2) (Fig 1A). Of note, the two most upregulated genes in Tpex were GZMK and IL-7R. We then performed differential abundance analysis to investigate differences in terms of clusters' frequencies across the three conditions (Res, NonRes, HD). At both timepoints Res had an increased frequency of Tpex and Teff CX3CR1pos compared to NonRes. Conversely, Term_exh2 cells were more abundant in NonRes (Fig. 1B). Next, we measured the magnitude of clonal expansion in antigen-experienced CD8 + T cells in Res and NonRes generating an overlay of the position of clonally expanded cells projected onto the UMAP. The most clonally expanded subsets were Tpex and Teff CX3CR1pos in Res (Fig. 1C) and Term_exh2 in NonRes (Fig. 1D) revealing a strong relationship between abundance and clonal expansion of the CD8 + T-cell subsets. Our scRNAseq results were then confirmed at the protein level with spectral flow-cytometry. The FlowSOM algorithm identified a CD8 + GZMK +CD127 + subset to be increased at baseline in Res vs NonRes (Fig. 1E). Remarkably, this cluster was also characterized by the expression of TIGIT, PD1 and TCF-1. These results were subsequently reproduced by manual gating of the GZMK +CD127 + subset which was significantly enriched (p<0.01) in Res vs NonRes (Fig. 1F). Of note, patients with a higher-than-median frequency of GZMK +CD127 +CD8 + T cells experienced significantly (p<0.02) prolonged overall survival after therapy (Fig. 1G).
Conclusion
Improving our understanding of the immune microenvironment in AML is critical for the rational integration of novel treatment strategies that seek to increase the response rate and/or maintain remission. We identified GZMK +IL7R + CD8 + cells as a distinct entity in the early differentiated CD8 + memory T cell pool that is clonally expanded and more abundant in Res compared to NonRes. This subset has a stem-like signature and may be associated with longer in vivo CD8 + T cell persistence and long-term AML control. An in-depth functional characterization with in vitro experiments and in vivo mouse models is currently ongoing.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal